Procare Health supports innovation at every stage of a woman’s life, and this is why all of our products are made from natural ingredients.
In the case of the menopause line, all the products have been developed under scientific rigor and focused on providing solutions to different woman’s needs at this stage of life. They are all supported by different clinical trials carried out in Spain:
An ongoing trial that has already shown some promising preliminary results in improving hot flashes, sleep quality, and overall quality of life after just one month.



OBSERVATIONAL, EXPLORATORY, PROSPECTIVE, NON-COMPARATIVE TRIAL
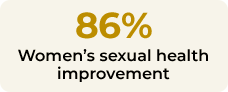
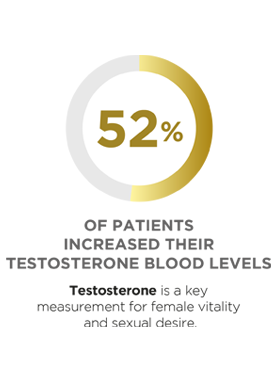
This trial assessed Libicare®´s efficacy in improving female sexual function in women in menopause and perimenopause with low sexual desire. Twenty-nine patients were treated with Libicare® for two months, and 86% of patients improved their overall FSFI (Female Sexual Function Index) score, suggesting a significant improvement in sexual function. Significant changes in hormones related to sexual desire were also observed after two months of treatment. Results of LIBIPIL were published in BMC Women’s Health.
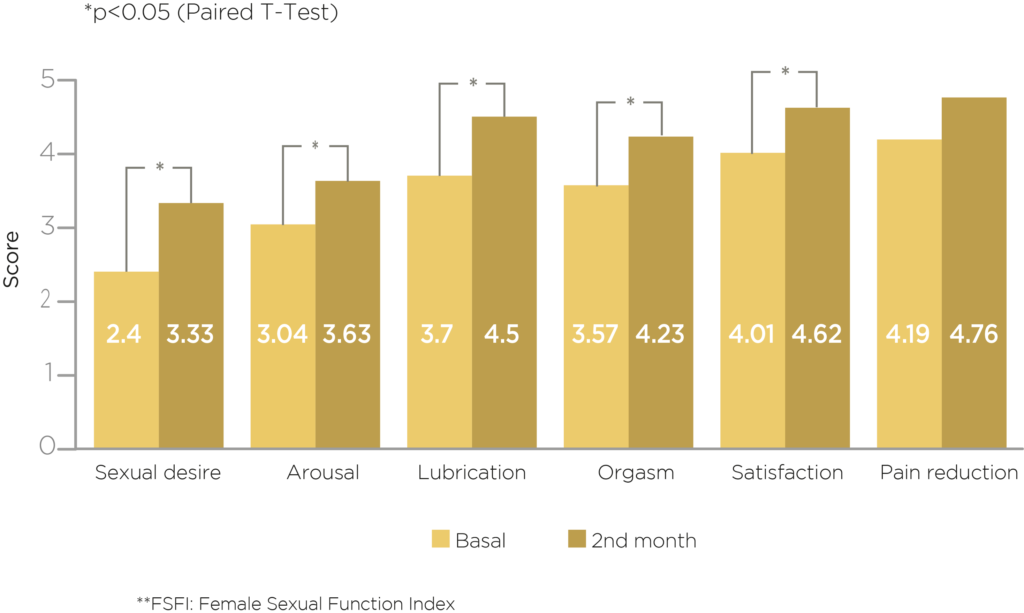
Libicare® Results in FSFI Score**
It improves sexual desire, arousal, lubrication, orgasm and pleasure in sexual relations


OBSERVATIONAL, PROSPECTIVE, MULTICENTRE, NON-COMPARATIVE TRIAL
This is a clinical trial performed to describe Libicare®´s efficacy in women aged 45 to 65 with decreased sexual desire and arousal in real life. It was based on the FSWB scale (Female sexual wellbeing scale), which measures sexual pleasure, arousal, orgasm, pain and trust between partners.
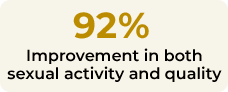
According to the trial’s results, 92% of patients improved their score in FSWB scale after 4 months, improving their sexual activity quality by using Libicare®.
According to the FSWB scale’s results, there was a reduction of 68% in sexual dysfunctions** after 4 months using Libicare®.
Según los resultados del estudio, el 92% de las pacientes aumentaron su puntuación en la escala EVAS-M a los 4 meses, aumentando la calidad de la actividad sexual con el uso de Libicare®
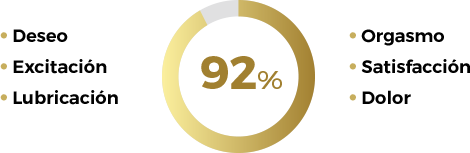
Escala EVAS-M: mide aspectos relacionados con la sexualidad: deseo, excitación, lubricación, orgasmo, dolor y satisfacción con su actividad sexual y con la pareja.
Según los resultados de la escala EVAS-M, con Libicare® se reducen las probables disfunciones sexuales** en un 68% a los 4 meses.
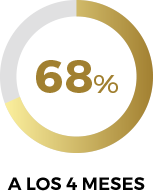
**Probable disfunción sexual: puntuación en la Escala EVAS inferior a 37,48

Escala EVAS-M: mide aspectos relacionados con la sexualidad: deseo, excitación, lubricación, orgasmo, dolor y satisfacción con su actividad sexual y con la pareja.
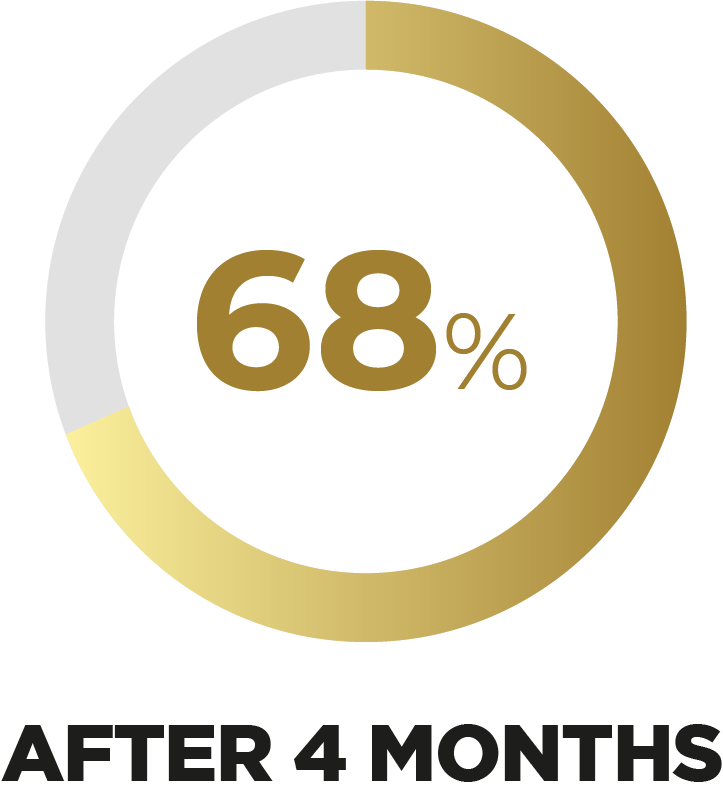
**Probable disfunción sexual: puntuación en la Escala EVAS inferior a 37,48
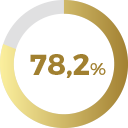
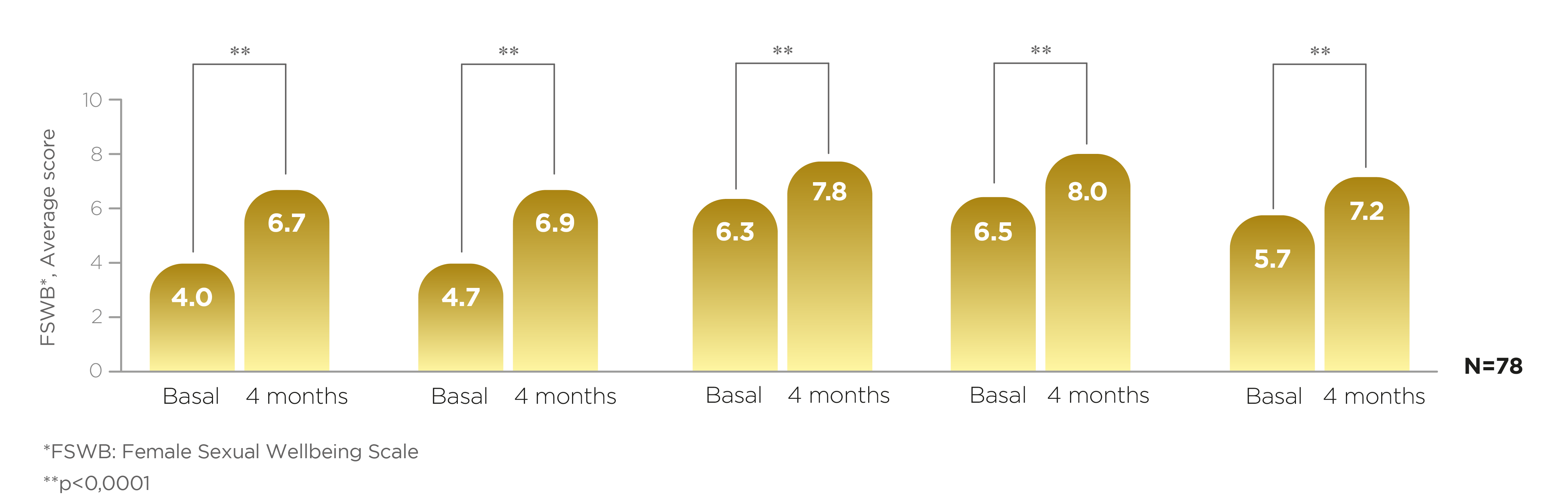
For each of the value domains in the FSWB scale the percentage of improvement in score after 4 months of using Libicare® tablets was:

of patients improved their score in
Sexual Desire
of patients improved their score in
Arousal
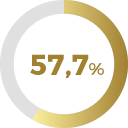
of patients improved their score in
Orgasm

of patients improved their score in
Pleasure

of patients improved their score in
Lubrication
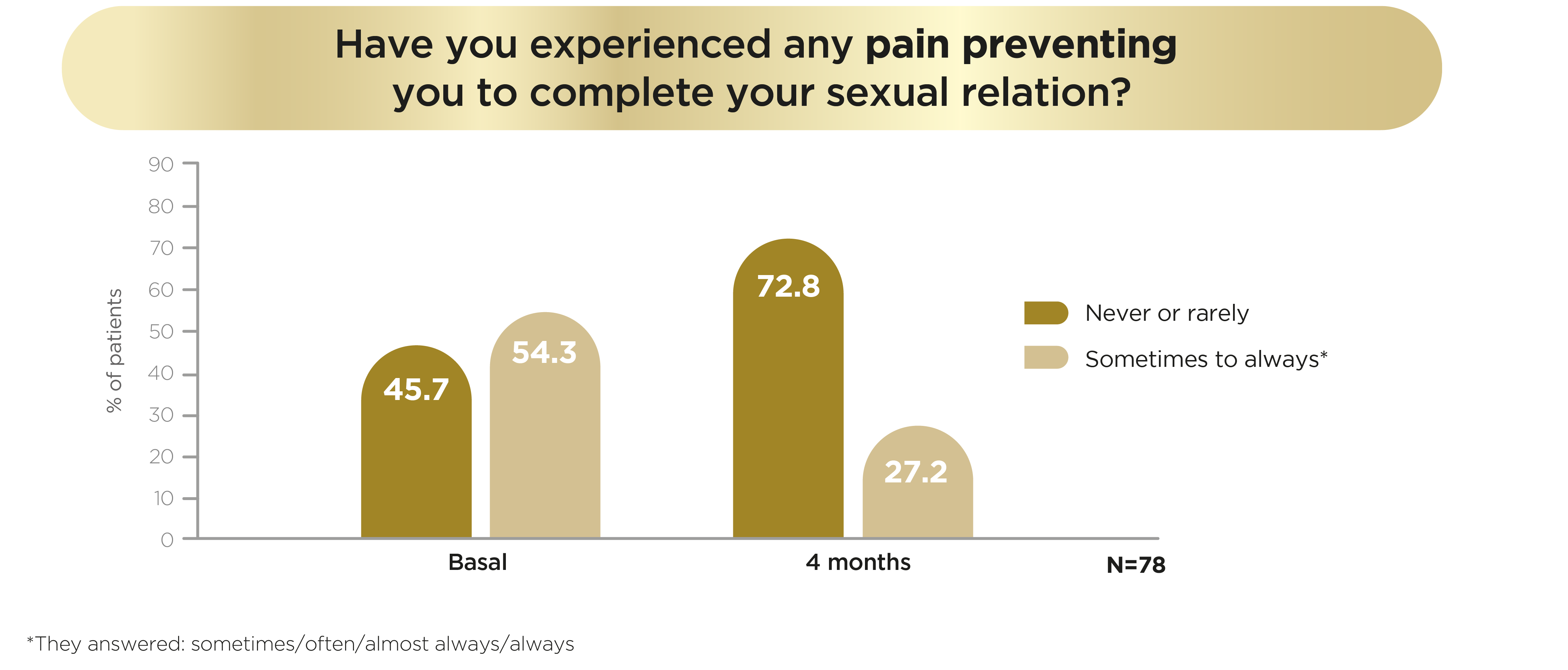
Additionally, according to LIBIDOBS study, dyspareunia was reduced using Libicare® tablets from 45% of patients never/rarely experiencing pain to 73% of patients at the end of the trial.
OBSERVATIONAL, PROSPECTIVE, MULTICENTER, NON-COMPARATIVE TRIAL
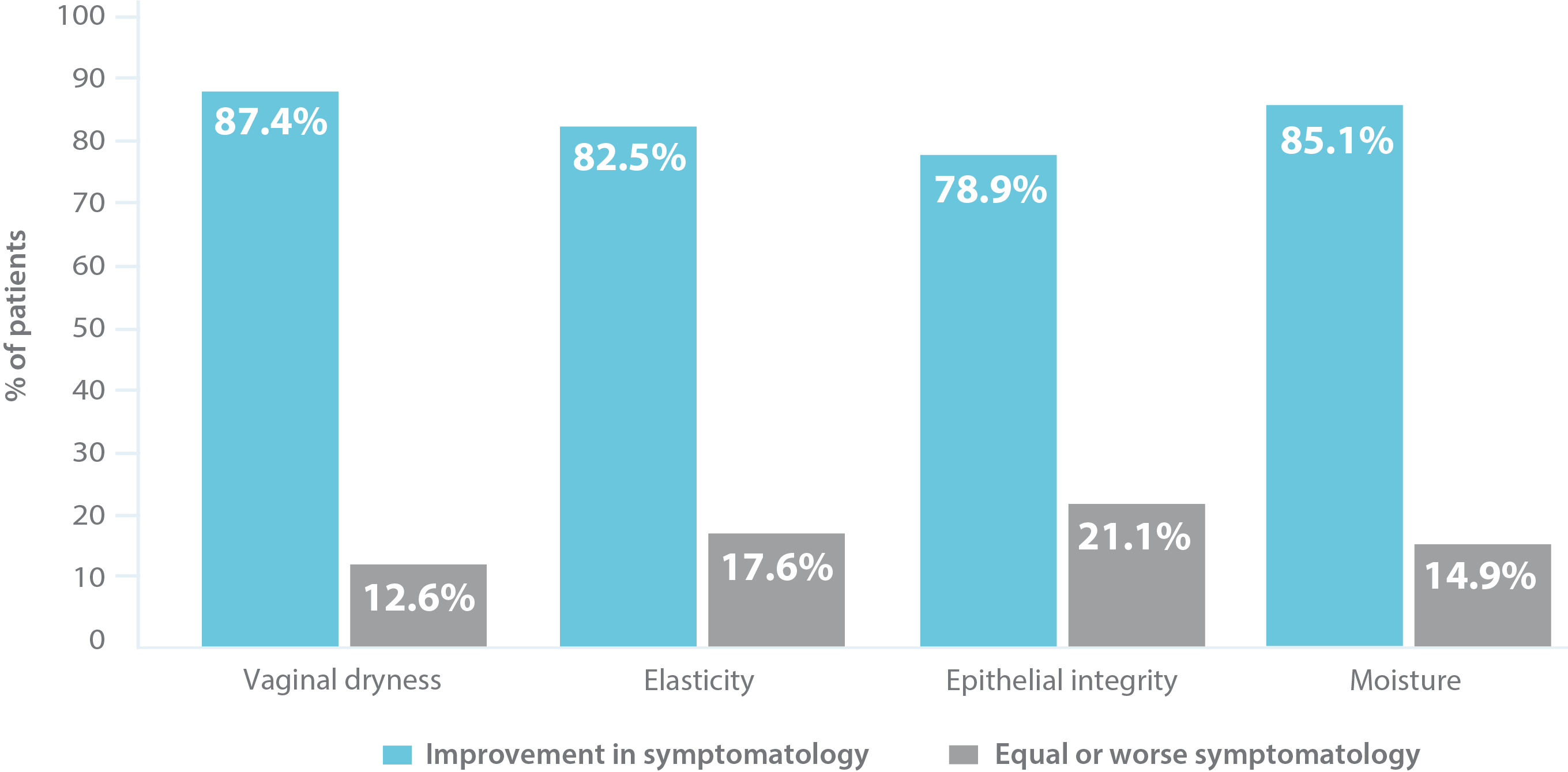
The objective of the Idra Study clinical trial is assessing safety and efficacy for the treatment of vulvovaginal atrophy (VVA) symptoms (dryness and/or dyspareunia) connected to menopause. 127 patients took part in the trial, with the following results:

Moisturizing efficacy


Of women experienced in pain relief during sexual relations (dyspareunia).
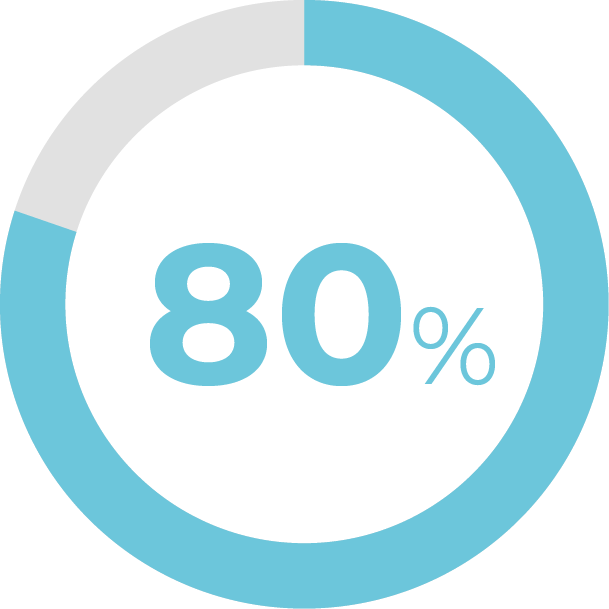
Of patients kept or improved their vaginal pH levels.

Of users were satisfied or very satisfied with Idracare after 3 months.